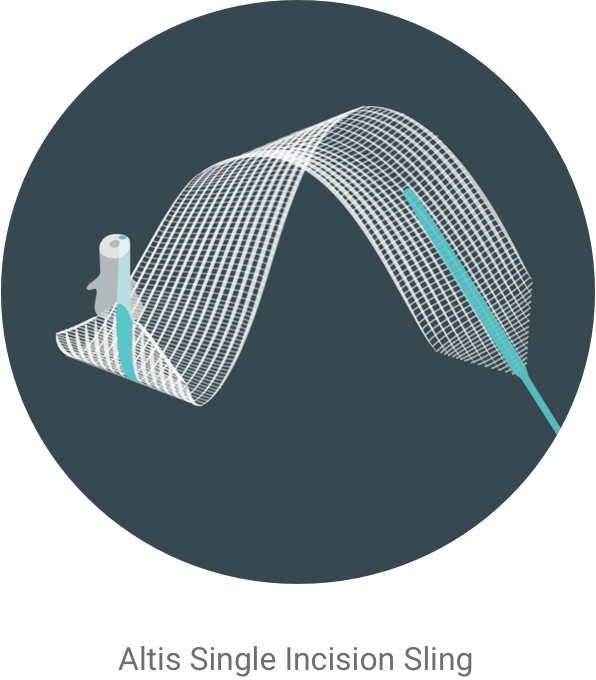
Relief from leaks
is possible
Approximately 78 million women in the U.S. suffer from urinary incontinence1 and of those, over 37% had Stress Urinary Incontinence (SUI).
Find your path to incontinence relief
No two people walk the same path to a diagnosis or a solution. Every woman’s experience with stress urinary incontinence (SUI) is different, and they may reach these steps at different paces and during different stages in their lives.
The right doctor will listen to you and put your needs first. But how do you find them?

Are you living with Stress Urinary Incontinence?
The muscles in your urethra work like a valve, opening and closing as needed to let urine out. But with stress urinary incontinence, also called SUI, the pelvic muscles that normally support the bladder and the urethra are weakened.
When this happens, urine leaks out of the bladder and can leave you feeling embarrassed, frustrated and unsure of what is happening to you.
Symptoms of stress urinary incontinence
Do you leak during any of the following activities?:
- Laughing
- Coughing
- Sneezing
- Heavy lifting
- Physical activity
- Sex
If you can say “yes” to one or more, you should talk to a doctor who is familiar with SUI and discuss a more permanent solution for treating urine leakage.

What causes SUI?
SUI can slowly develop as you age and may also be the result of a specific event such as childbirth, or be a result of smoking, obesity or other previous tissue traumas in the area.2
What are your treatment options?
You name it, you’ve tried it. From pads, diapers, special underwear and Kegels – some of it helps, but doesn’t relieve the burden of SUI. You deserve a treatment that addresses the source of the problem, not something that just provides a temporary fix for symptoms.

Avoiding and covering up leaks only adds to the burden of having SUI in the first place. A lasting solution, with minimal downtime, can help relieve the added burden of constantly hiding your SUI symptoms.
Luckily, there’s Altis® Single Incision Sling.
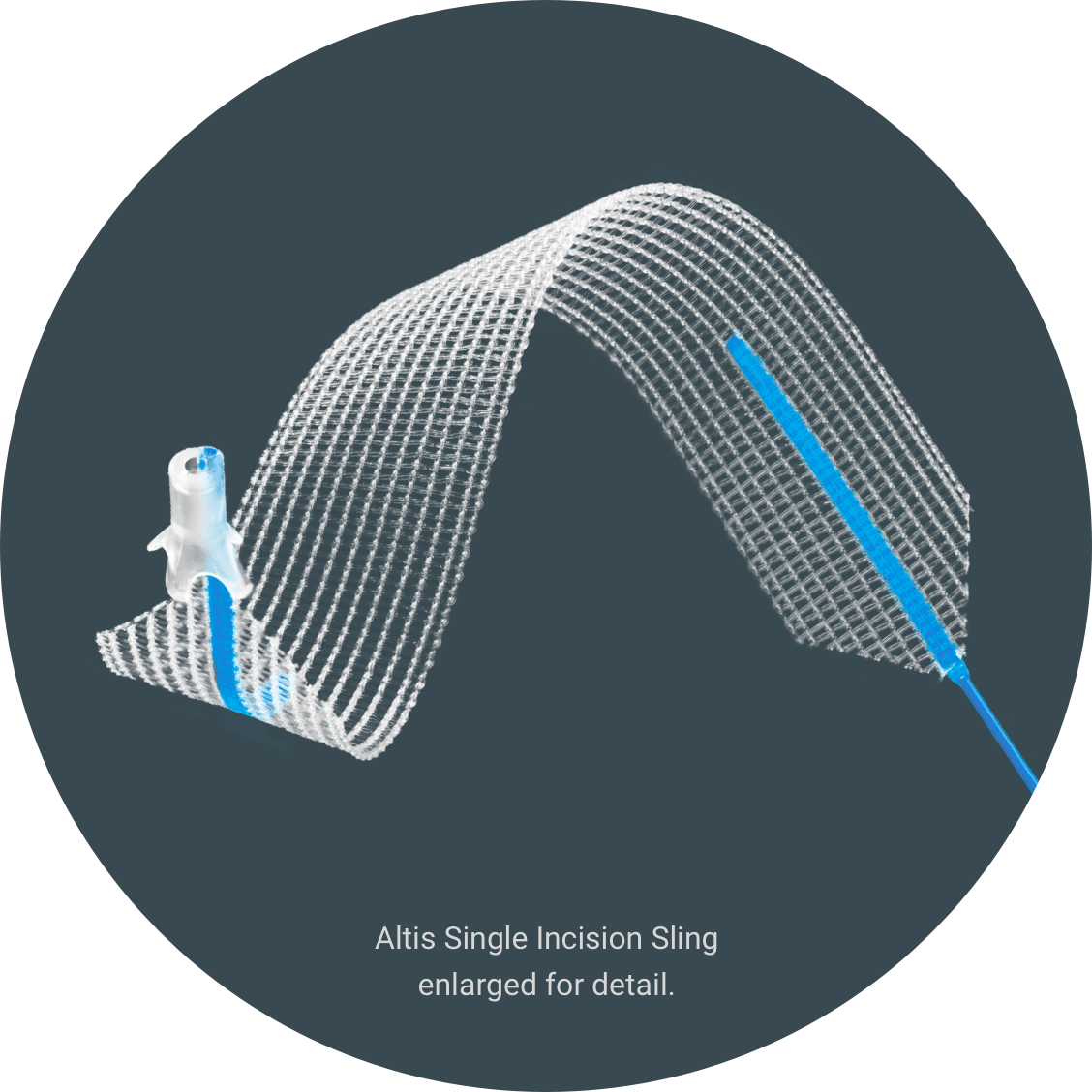
Introducing Altis®
Women experiencing SUI deserve a clinically proven, effective solution. Altis is a treatment backed by clinical studies with firsthand testimonials from women who’ve had the procedure with successful outcomes.
Altis is a sling that treats stress urinary incontinence by supporting the urethra to keep it in its correct position.3 The procedure is a minimally invasive4 outpatient surgery.3
What have others experienced?
0 %
saw a median reduction in pad weight4
0 %
reported feeling “very much better” or “much better” improvements after their surgery at 24 months4
0 %
reported no urine leakage related to SUI at 24-months post procedure4
Find a specialist
Locate a pelvic surgery specialist near you

Take back your normal
Locating a doctor with the expertise and empathy to treat your condition is key. You’re probably wondering, “Is there a pelvic surgical specialist near me?” We’ve got that covered.
Simply enter your ZIP code to see a listing of pelvic doctors in your area. Each listing provides the doctor’s name and area of pelvic health expertise, along with their website, phone number and distance from your home.
This directory was created and is maintained by Coloplast. It is intended to support patients who want to learn more about treatment options for stress urinary incontinence (SUI) and pelvic organ prolapse (POP) by helping connect them to physicians who have demonstrated qualifications and interest in providing quality care for these conditions. Names and details in this directory are provided for your information only. Decisions regarding choice of physician and treatment are a patient’s responsibility, as is all communication and interaction with listed medical professionals. Any information you send to a physician is not covered by the Coloplast Privacy Policy.
Physicians listed on the Physician Locator pay no fee for inclusion. Some physicians on this list may purchase products from, provide consulting services to and/or be a party to a co-marketing agreement with Coloplast. Coloplast makes no representations or warranties regarding, and shall not be responsible for, the competencies or skill level of any of the physicians listed on the Physician Locator or the quality of their procedural outcomes. You and your physician must determine the right procedure for you.
For more information about this physician finder or if you have questions about how to add or manage directory listings please contact the administrator.
Important safety information
Patient Materials (Direct to Consumer)
Important Safety Information:
Stress urinary incontinence is a condition in which urine involuntarily leaks out of the urethra (the tube that brings urine from the bladder to the outside of the body) during times of high pressure such as coughing, sneezing or exercising. Stress urinary incontinence can be treated with a surgical procedure in which an incontinence sling is implanted to support the urethra. An incontinence sling is intended to provide support to the urethra to help stop urine from leaking and to help control when urine is emptied from the bladder.
The Altis Single Incision Sling System is indicated for the treatment of female stress urinary incontinence (SUI) resulting from the urethra not closing properly (urethral hypermobility) and/or weakness of the urethral sphincter (intrinsic sphincter deficiency (ISD).
The Altis Single Incision Sling System is not for females who have the following: are pregnant or have desire for future pregnancy, potential for further growth (e.g., adolescents), known active urinary tract infection and/or infection in operative field, taking blood thinning medication (anti-coagulant therapy), abnormal urethra (e.g., fistula, diverticulum), intraoperative urethral injury, any condition, including known or suspected pelvic pathology, which could compromise implant or implant placement, and sensitivity/allergy to polypropylene. Check with your Physician on the warnings, precautions and risks associated with the use of this mesh sling.Check with your Physician on:
- Alternative incontinence treatments that may be appropriate
- The reason for choosing a mesh sling procedure
- The postoperative risks and potential complications of transvaginal mesh sling surgery
- The mesh sling to be implanted is a permanent implant
- Some complications associated with the implanted mesh sling may require additional surgery; repeat surgery may not resolve these complications
- Serious adverse tissue responses or infection may require removal of parts of the mesh sling, or the entire mesh sling, and complete removal of the mesh sling may not always be possible
- Individuals who have varying degrees of collagen laydown that may result in scarring
- Certain underlying conditions may be more susceptible to postoperative bleeding, impaired blood supply, compromised/ delayed healing, or other complications and adverse events, as with all surgical procedures
You should consider the risks and benefits of the Altis Single Incision Sling System.
Any future pregnancy could negate the benefits of this mesh sling surgical procedure.
You should report any bleeding, pain, abnormal vaginal discharge or sign of infection that occur at any time.
A mesh sling is implanted inside the vagina to support the urethra. The operation to place a mesh sling is considered major surgery.
A mesh sling procedure is a surgical solution that has risks such as: mesh extrusion, pelvic/urogenital pain, groin pain, hip pain, urinary retention, bleeding, new onset (de novo) urgency, delayed wound healing, painful intercourse (dyspareunia), inflammation, nausea, overactive bladder, pain, pelvic hematoma, reaction to antibiotic, slight discomfort upon return to work, urinary tract infection, urine stream decreased, and voiding dysfunction.
Adverse events are known to occur with transvaginal synthetic mesh sling procedures and implants. Adverse events following mesh implantation may be new onset (de novo), persistent, worsening, transient, or permanent.
Additional potential complications include, but are not limited to: abscess (acute or delayed), adhesion/scar formation, allergy, hypersensitivity or other immune reaction, bleeding, hemorrhage or hematoma, dehiscence, delayed wound healing, extrusion, erosion or exposure of mesh sling into the vagina or other structures or organs, fistula formation, infection, inflammation (acute or chronic), local irritation, necrosis, new onset (de novo) and/or worsening painful intercourse (dyspareunia), neuromuscular symptoms (acute or chronic), pain, partner pain (acute or chronic) and/or discomfort during intercourse, perforation or injury of soft tissue (e.g., muscles, nerves, vessels), structures, or organs (e.g., bone, bladder, urethra, ureters, vagina), seroma (pocket of fluid build-up), sling migration, suture erosion, bladder storage dysfunction (e.g., increased daytime frequency, urgency, nocturia, overactive bladder, urinary incontinence), ureteral obstruction, urinary tract infection, voiding symptoms (e.g., painful urination (dysuria), urinary retention, incomplete emptying, straining, positional voiding, weak stream), granulation tissue formation, palpable mesh (patient and/or partner), sexual dysfunction, vaginal discharge (abnormal) and vaginal scarring or tightening.
The occurrence of these events may require one or more revision surgeries, including removal of the mesh sling.
Complete removal of the mesh sling may not always be possible, and additional surgeries may not always fully correct the complications.
There may be unresolved pain with or without mesh sling explantation.
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a mesh sling is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Minneapolis, MN
PM-03328 02/2021
References:
- Patel, U. J., Godecker, A. L., Giles, D. L., & Brown, H. W. (2022). Updated Prevalence of Urinary Incontinence in Women: 2015-2018 National Population-Based Survey Data. Female pelvic medicine & reconstructive surgery, 28(4), 181–187. https://doi.org/10.1097/SPV.0000000000001127
- Abrams, P., Andersson, K. E., Apostolidis, A., Birder, L., Bliss, D., Brubaker, L., Cardozo, L., Castro-Diaz, D., O’Connell, P. R., Cottenden, A., Cotterill, N., de Ridder, D., Dmochowski, R., Dumoulin, C., Fader, M., Fry, C., Goldman, H., Hanno, P., Homma, Y., Khullar, V., … members of the committees (2018). 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: EVALUATION AND TREATMENT OF URINARY INCONTINENCE, PELVIC ORGAN PROLAPSE AND FAECAL INCONTINENCE. Neurourology and urodynamics, 37(7), 2271–2272. https://doi.org/10.1002/nau.23551
- Surgery for stress urinary incontinence. ACOG. (n.d.). Retrieved December 2, 2022, from https://www.acog.org/Patients/FAQs/Surgery-for-Stress-Urinary-Incontinence
- Kocjancic, E., Erickson, T., Tu, L. M., Gheiler, E., & Van Drie, D. (2017). Two-year outcomes for the Altis® adjustable single incision sling system for treatment of stress urinary incontinence. Neurourology and urodynamics, 36(6), 1582–1587. https://doi.org/10.1002/nau.23156