Surgical solutions for prolapse
Restore your normal

It’s time to get proactive about your prolapse
Prolapse can be repaired rather than just managed. And following surgery many women ultimately realize, “I wish I would’ve done it sooner.” It’s time you choose a solution that treats the source of the problem, instead of masking its symptoms. It’s time to find lasting relief.
Reconstructive surgical solutions are effective in repairing POP at the source of the symptoms, so you can get back to living the life you want.
Different prolapse procedure types
There are two approaches to POP repair.
Your physician will recommend a procedure based on your type of prolapse and your medical history.
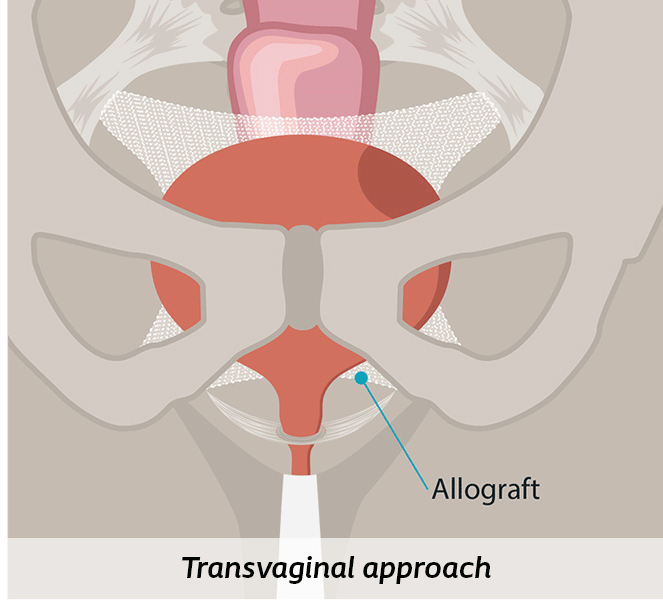
Through the vagina (Transvaginal)
This approach includes various types of procedures where a surgeon corrects your prolapse through your vagina using your own tissue or a biologic graft, called an allograft (colpopexy).1
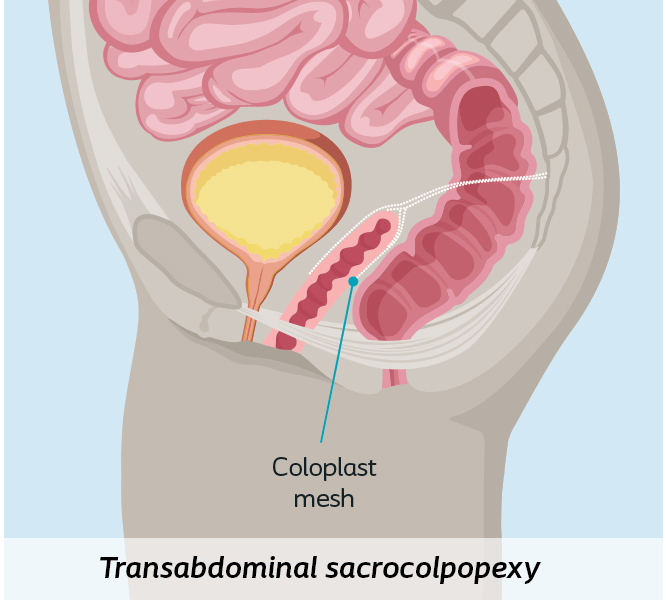
Through the abdomen (Transabdominal)
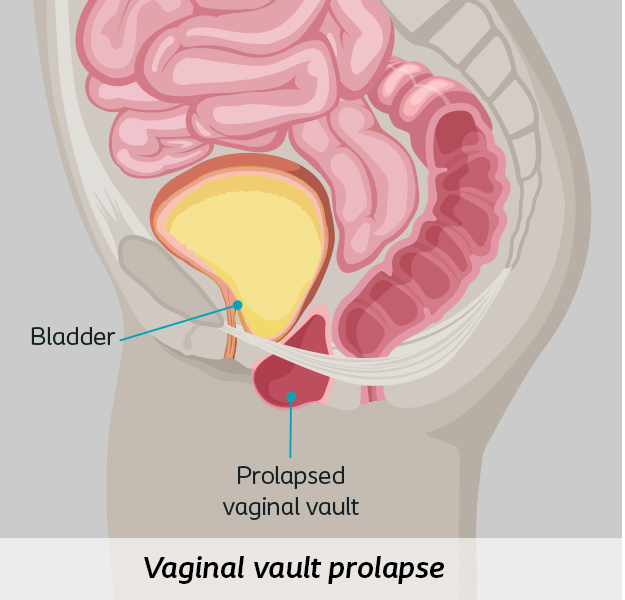
This approach corrects prolapse via incisions in the abdomen that allow surgeons to attach surgical mesh from the vagina to tissue at the sacrum (sacrocolpopexy). This procedure can be done using a surgical robot or laparoscopic instruments.1

What to expect before,
during and after surgery
Before surgery
You will likely have a pre-op visit with your doctor and possibly with your anesthesiologist. This is the time to ask questions you may have about your surgery and post-operative care. Your surgeon will review your medical history and recent experiences. You will have to provide a list of all medication and supplements you take. They will inform you of the correct preparations you should take prior to your procedure and medically clear you for your surgery.
Some physicians might recommend a “bowel prep” for the day before your procedure (a laxative or an enema). And if you are going to have anesthetic, you’ll be instructed not to eat or drink anything a minimum of six hours before your surgery.2
During surgery
During the surgery, your surgeon will place the synthetic graft (mesh) through an incision in your abdomen. If your surgeon uses a biologic graft (allograft) they will place it through an incision in the vagina.
If a synthetic mesh is used, your body’s tissue will grow through the holes in the mesh and the mesh will slowly become part of your body. Biologic grafts are typically absorbed by your body within 6 to 9 months.3
After surgery and into recovery
Although every pelvic organ prolapse patient’s recovery process is different, there are general, recovery guidelines that apply to most pelvic floor procedures.1
- It is common to spend one to four days in the hospital, depending on the type of surgery performed (outpatient or a brief stay in hospital).
- Physical strain, sexual intercourse, and heavy lifting should typically be avoided for six weeks after surgery.
- Patients often can resume other normal activities after two weeks or at the physician’s discretion.
Your physician will provide you with a specific details about your recovery process, and they may have other recommendations based on your individual needs.
Follow your physician’s recovery directions carefully. Even though you may not be feeling much pain from your surgical procedure, your body needs time to heal properly from the surgery and allow the mesh material to incorporate within your body’s natural tissue.
Contact your physician immediately if you have any problems after your surgery. It’s important to tell them if you have excessive bleeding, pain, abnormal vaginal discharge or signs of infection occurring at any time during your recovery. Your physician will give you a more detailed list of possible adverse events that can happen. Continue with your annual and other routine check-ups and follow-up care.


What are the outcomes?
Women experiencing POP deserve a clinically proven and effective solution that lasts.
Prolapse repair procedures have effective outcomes such as:
0 %
of patients were “satisfied” or “very satisfied”4
0 %
of patients stated they would definitely “do it all over again” if they had the chance4
0 %
stated they “would definitely recommend to a friend”4

Let’s talk about mesh
Pelvic mesh is a woven sheet of synthetic
material designed to support organs via surgery.5
In 2019, the US Food and Drug Administration (FDA) ordered the sale and distribution of a specific type of synthetic mesh (known as transvaginal mesh) to be stopped.6 Similarly, POP surgeries involving synthetic mesh are no longer performed through the vagina (transvaginally), rather they occur through the patient’s abdomen (transabdominally). Learn more about synthetic mesh on the FDA website.
For over 15 years, transabdominal mesh has shown to be safe for POP repairs, with low rates of complications.7 Transabdominal mesh was created specifically for women’s bodies and is composed of a very thin, lightweight mesh made from a soft synthetic material designed to feel more natural.
FAQs
Prolapse 101
What is pelvic organ prolapse?
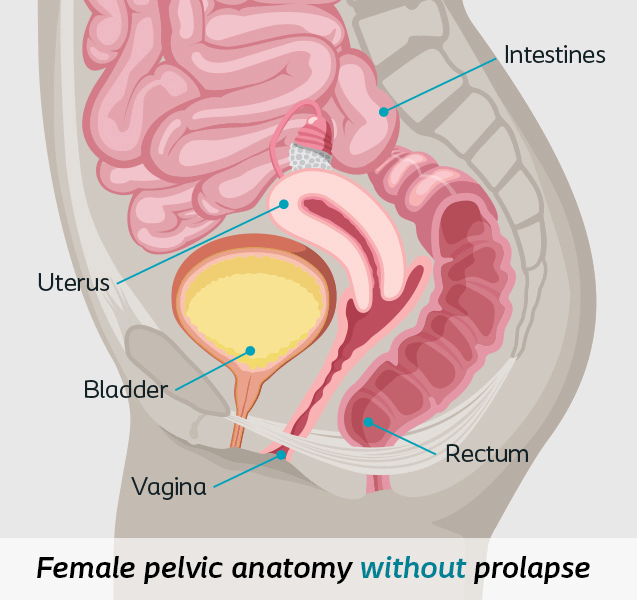
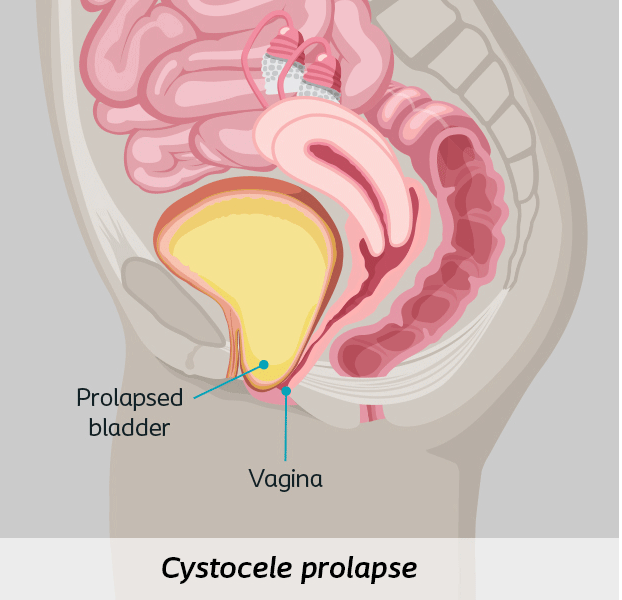
Pelvic organ prolapse (POP) is the dropping of the pelvic organs caused by the loss of normal support of the vagina. It occurs when there is weakness or damage to the normal support of the pelvic floor causing pelvic organs (the vagina, cervix, uterus, bladder, urethra, intestines or rectum) to drop down. Women with POP may feel or see a bulge coming out of the opening of their vagina.8
POP
pelvic organ prolapse is also known as “POP”
What are the symptoms of pelvic organ prolapse?
While some women do experience symptoms related to pelvic organ prolapse, many others may not notice any symptoms at all. It may also be difficult to identify prolapse symptoms as they often progress very slowly and you may not notice changes until you become symptomatic.9
Symptoms will often depend on which type of prolapse you are experiencing and the specific organ that is descending. Some common prolapse symptoms include pressure or fullness in the pelvic area, backaches, painful intercourse, urinary problems and constipation.9
Learn more about common symptoms of prolapse.
How common is pelvic organ prolapse?
Up to 50% of women will experience pelvic organ prolapse.10
What are the different types of pelvic organ prolapse?
There are three categories and five types of pelvic organ prolapse.9
- Anterior Vaginal Wall Prolapse or cystocele
- Posterior Wall Prolapse includes rectocele or enterocele
- Apical Prolapse includes vaginal vault or uterine prolapse
Find out more about each type of prolapse and their signs and symptoms here.
What causes pelvic organ prolapse?
Pelvic organ prolapse can develop when events or activities happen that lead to increased pressure on the pelvic floor. Pregnancy and childbirth are the most common causes of pelvic organ prolapse due to the increased stress placed on the pelvic floor muscles.8
However, menopause, genetics, lifestyle (smoking), chronic constipation, previous vaginal surgery, chronic coughing or straining, heavy lifting, obesity and many other factors are connected to pelvic organ prolapse.8
Learn more about the causes of POP here.
Prolapse surgical treatment options
Can pelvic organ prolapse be successfully treated?
Yes, pelvic organ prolapse can be treated successfully.11
There are many different treatment options available for pelvic organ prolapse. It is important to discuss your individual situation with a pelvic floor specialist who will discuss what options may be appropriate for your specific situation.
What are the treatment options for pelvic organ prolapse?
There are both non-surgical and surgical treatment options. The non-surgical options may include lifestyle changes, Kegel exercises, or vaginal pessaries (a removable device).11
Depending on the severity of your pelvic organ prolapse symptoms and general health, surgery may be recommended. Surgical treatments are designed to repair the prolapse and support the pelvic organs with a biologic graft or synthetic mesh.11
There are different types of pelvic organ prolapse surgeries, so it is important to discuss your options with your physician in detail to ensure that you find a treatment option that is right for you.
What is prolapse surgery?
Prolapse surgery is a surgical procedure that uses a biologic graft or a synthetic mesh, and suturing it into place to repair the prolapse. The goal is to help keep the pelvic organs supported and in the correct place. The procedure can be performed through small incisions in either the vagina or abdomen.11
How long does it take to recover from prolapse surgery?
Every patient’s recovery time is different. It is generally recommended that physical strain, sexual intercourse and heavy lifting should be avoided for six weeks after surgery, but the patient may resume other normal activities after two weeks or at the surgeon’s discretion. However, each patient and surgery is different. Your doctor will be able to provide you with your own individual recovery plan.
What are the risks associated with prolapse surgery?
Every surgery carries some level of risk. Prolapse repair surgery may not be suitable for every patient, and a thorough discussion between you and your doctor will enable both of you to determine if this treatment is right for you.
This surgery is not suitable for patients with the following:
- Pregnancy or desire for future pregnancy
- Potential for further growth (e.g. adolescents)
- Pre-existing local or systemic infection
- Taking anticoagulant therapy
- Any condition, including known or suspected pelvic pathology, which could compromise implant or implant placement
- Sensitivity/allergy to polypropylene
Other potential complications include pain, bleeding, infection, injury to blood vessels or nerves, bladder, urethra or bowel injury during mesh placement—which may require additional surgery. Complications can be temporary or permanent. For a detailed list of the potential risks please see the Important Safety Information.
Ask your doctor for more information about potential risks and complications, as well as your specific treatment plan.
What type of doctor performs the surgery?
Typically, prolapse repairs are performed by urogynecologists, urologists, or gynecologists.
Learn more about finding the right doctor to fit your needs.
Prolapse surgical treatment cost and coverage
How much does this cost? Will insurance cover the prolapse procedure?
Most insurance plans, including Medicare, cover these procedures. Consult your insurance carrier to find out the specific criteria for coverage. The reimbursement specialist at your physician’s office may also be able to help you get answers.